When it comes to measuring specific ion concentrations in water, soil, and biological samples, an ion selective electrode (ISE) is one of the most reliable instruments in analytical chemistry. HORIBA, a global leader in electrochemical measurement, offers a comprehensive range of combination ISEs designed for high accuracy, long-term stability, and ease of use across a wide range of applications.
instruments in analytical chemistry. HORIBA, a global leader in electrochemical measurement, offers a comprehensive range of combination ISEs designed for high accuracy, long-term stability, and ease of use across a wide range of applications.
What is an Ion Selective Electrode?
An ion selective electrode measures the concentration of a particular ion in a solution by detecting the potential difference between two electrodes — an ion selective electrode and a reference electrode. When both electrodes are immersed in the sample and connected to an ion meter, the potential difference produced corresponds directly to the ion concentration in the sample.
This technique, known as the ion electrode method, is widely used in laboratories, environmental monitoring, food testing, and clinical analysis because it provides fast, direct concentration readings without the need for complex chemical reactions.
How Combination ISEs Work
In traditional setups, the ion selective electrode and the reference electrode are separate components. HORIBA’s combination ISEs integrate both into a single, compact probe, making them more convenient and easier to handle in routine testing environments.
Each electrode within the combination probe has a distinct function:
| Electrode Type | Function |
|---|---|
| Ion Selective Electrode | Generates an electromotive force through its selective membrane, based on the difference in ion concentration between the internal solution and the sample solution. |
| Reference Electrode | Maintains a stable potential regardless of changes in the sample or standard solution composition. |
The following figure shows the structure of a combination type ion selective electrode where two electrodes are integrated.
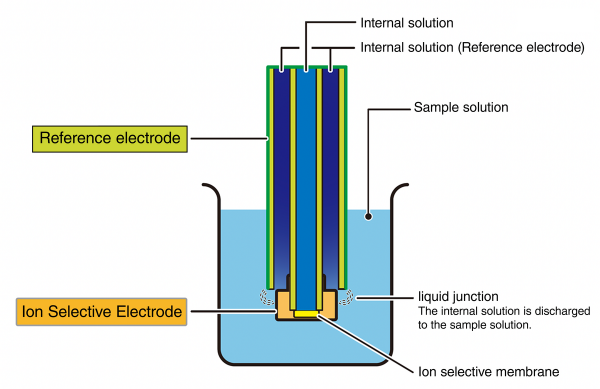 This design ensures reliable and consistent measurements, even when working with complex sample matrices.
This design ensures reliable and consistent measurements, even when working with complex sample matrices.
The Role of the Reference Electrode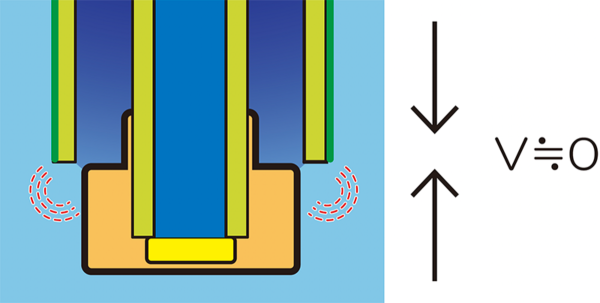
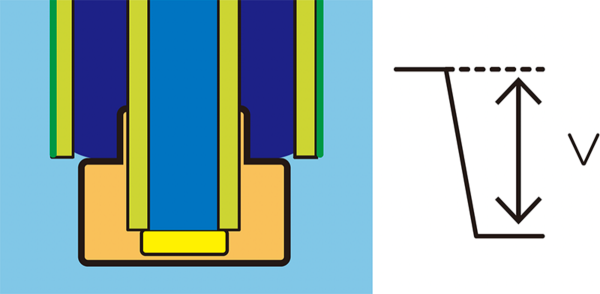
A key feature of HORIBA’s ISE design lies in the stability of the reference potential. The reference electrode generates a fixed potential used as a baseline for comparison against the ion selective electrode’s potential. For accurate readings, this reference potential must remain stable and unaffected by the sample composition.
This stability is achieved through the discharge of the internal solution from the reference electrode into the sample. The controlled diffusion of this internal solution helps maintain a near-constant potential (V ≅ 0), ensuring minimal variation and highly reproducible results.
 However, if the internal solution is insufficient, or if the electrode’s rubber stopper remains closed (preventing the internal solution from being exposed to the atmosphere), the measurement accuracy may decline. This can cause the reference potential to drift, leading to unreliable results. Regular maintenance and correct electrode handling are therefore essential to maintain precision.
However, if the internal solution is insufficient, or if the electrode’s rubber stopper remains closed (preventing the internal solution from being exposed to the atmosphere), the measurement accuracy may decline. This can cause the reference potential to drift, leading to unreliable results. Regular maintenance and correct electrode handling are therefore essential to maintain precision.
Calibration and Temperature Control
Before measurement, ISEs must be calibrated using standard solutions. Calibration allows the electrode to convert voltage readings directly into ion concentration values. Because electrode response is temperature dependent, measurements should ideally be performed under stable temperature conditions to ensure accuracy.
HORIBA Combination ISE Lineup and Applications
HORIBA’s range of combination ion selective electrodes is designed to cover a wide variety of ions found in agricultural, industrial, and environmental settings. Each model offers dependable performance, robust construction, and easy integration with HORIBA ion meters.
5002S-10C Ammonia (NH₃) Electrode
Ideal for detecting ammonia levels in agriculture, fish tanks, seawater, wastewater, power station water, and biological samples. It’s also used in plating baths, air/stack gas testing, and environmental monitoring applications.
6583S-10C Calcium Ion (Ca²⁺) Electrode
Commonly used in soil, plant tissue, and agricultural testing, this electrode is also suitable for water softening systems, boiler feed water, and mineral water analysis. It plays an important role in clinical, dental, food, and dairy testing where calcium levels are monitored.
6560S-10C Chloride Ion (Cl⁻) Electrode
A versatile electrode used in river and tap water, soils, boiler feed water, cement production, clinical analysis, and plating baths. It’s also frequently used in food, dairy, and beverage quality control as well as sweat and urine testing.
6561S-10C Fluoride Ion (F⁻) Electrode
Designed for drinking water, wastewater, and dental product testing (toothpaste and mouthwash). It’s also used in air quality monitoring, acid analysis, food, plant tissue, and biological fluids. Even in coal, carbonated beverages, and bone research.
6581S-10C Nitrate Ion (NO₃⁻) Electrode
Used extensively in agriculture, plant tissue, and soil analysis to monitor nitrate levels. It’s also ideal for river water, tap water, and clinical analysis (sweat and urine). As well as food, beverage, and plating bath testing.
6582S-10C Potassium Ion (K⁺) Electrode
An essential tool for measuring potassium in fertilizers, wastewater, drinking water, clinical samples (saliva and serum), plant tissue, and food or beverage products such as wine and dairy.
Precision You Can Rely On
Whether monitoring nutrients in soil, assessing drinking water safety, or conducting clinical testing, HORIBA’s combination ISEs deliver fast, precise, and stable ion concentration measurements. Backed by HORIBA’s long-standing expertise in electrochemical technology, these electrodes are built for consistent results and long service life — making them a trusted choice in laboratories and field applications worldwide.