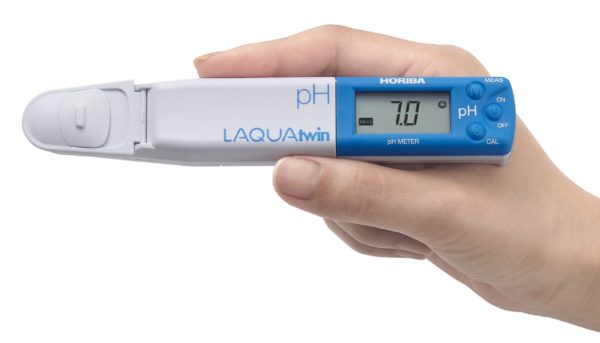
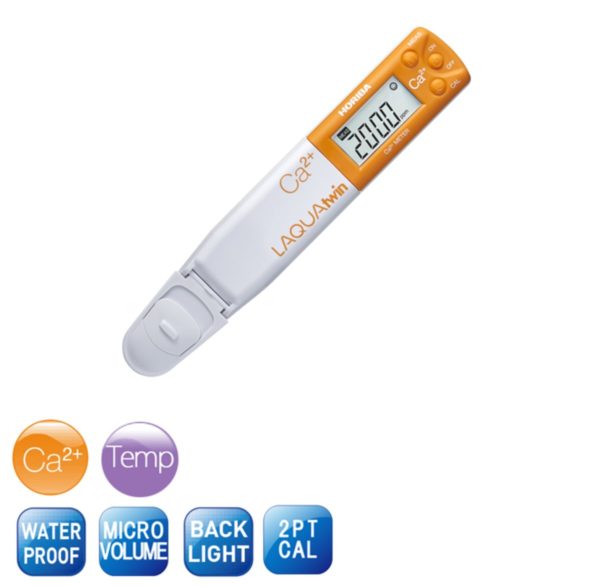
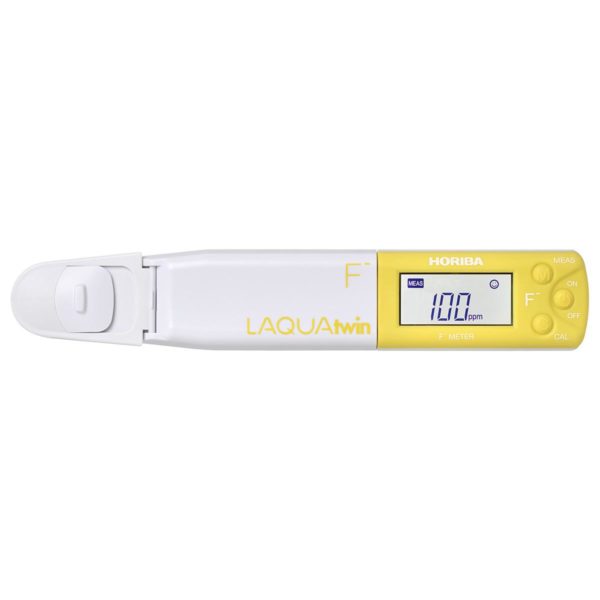
pH plays a powerful role in everything from growing veggies to making your wine taste just right.
Let’s break down how this one little number impacts our everyday lives.
 🌱 In the Garden:
🌱 In the Garden:
Soil isn’t just dirt—it’s a complex living system. And one of the biggest factors influencing how well plants grow is soil pH. Get it wrong, and you might be starving your plants of key nutrients or even poisoning them with metals like aluminum.
-
Acidic soil can make minerals more soluble, releasing toxic metals and locking up essential nutrients like phosphorus.
-
Alkaline soil often lacks nutrients like zinc, copper, and iron, which plants need to thrive.
Most plants are happiest in a range of 6.0 to 7.0, where nutrients are most available. But there are exceptions—blueberries prefer more acidic conditions, while hyacinths lean slightly alkaline.
Good news: you can tweak your soil’s level by adding materials like lime (to raise it) or sulfur (to lower it). But before you start sprinkling, a soil test will help you figure out exactly what’s needed.
 🐟 Water Life: Aquaculture and Ecosystems
🐟 Water Life: Aquaculture and Ecosystems
Fish aren’t just picky about temperature—they’re sensitive to pH too. A water source that’s too acidic or too basic can stress or even kill aquatic life.
-
Below 5, fish reproduction can be affected, and young fish are especially vulnerable.
-
Above 9, harmless ammonium turns into toxic ammonia—bad news for fish, especially in warmer waters.
A safe range for most aquatic species is between 6.5 and 9.0, and buffering agents like bicarbonates help keep that range stable in controlled environments like aquariums.
 🚰 Clean and Safe: Water Treatment
🚰 Clean and Safe: Water Treatment
When it comes to drinking water, pH isn’t just about taste—it’s a safety issue.
-
Low pH water (under 6.5) can corrode pipes, leaching metals like copper and lead into the water.
-
High pH water (above 8.5) tastes off and can reduce the effectiveness of disinfectants like chlorine.
In wastewater treatment, keeping the these levels in check ensures that chemical and microbial processes run smoothly. Operators are constantly adjusting the pH to optimize treatment and protect the environment.
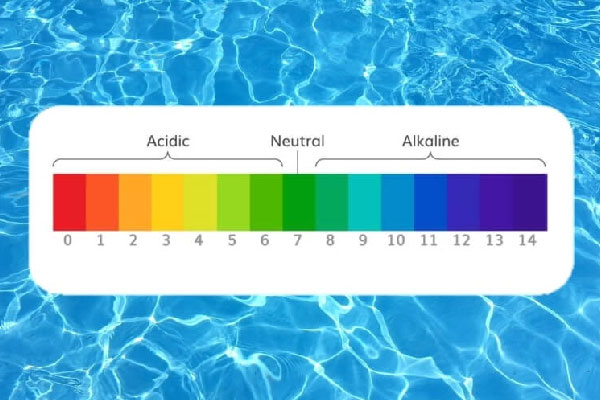 🏊 Perfect Pools:
🏊 Perfect Pools:
A sparkling blue pool is inviting—unless the pH is off.
-
Too high, and chlorine can’t do its job, leaving the pool vulnerable to algae and bacteria.
-
Too low, and swimmers might experience eye and skin irritation. Worse, acidic water can damage pool surfaces and plumbing.
The sweet spot? 7.2 to 7.8. That keeps the water comfy and safe, and your pool sparkling clean.
 🧀 In the Kitchen: Safety
🧀 In the Kitchen: Safety
Regarding food, pH is essential for everything from taste to safety to shelf life.
-
Milk and cream are tested for to detect spoilage or infection.
-
Cheese texture and butter flavor are both influenced by pH.
-
Yogurt relies on a low pH to support the good bacteria that make it tangy and thick.
In baking, acidifying dough helps preserve bread. Canned food should have a pH below 4.6. This crucial to prevent dangerous bacteria like Clostridium botulinum from growing.
A too-high pH in meat? That could be a sign it’s gone bad. Yuck.
 🍷 Fermented Fun: Brewing and Winemaking
🍷 Fermented Fun: Brewing and Winemaking
pH control is key in brewing and winemaking, where microbes play a starring role.
-
In beer, mash pH (ideally 5.3 to 5.8) helps enzymes do their job, affecting flavor and clarity.
-
In wine, low pH helps prevent spoilage and creates that crisp, dry taste. Most wines sit between pH 3.0 and 4.0, with white wines usually being more acidic than reds.