pH in Soil and Nutrient Availability
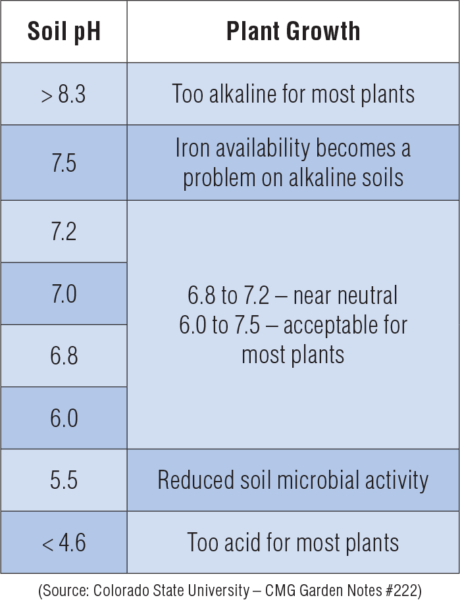
The desirable range for optimum plant growth varies among crops. Generally, soil pH 6.0-7.5 is acceptable for most plants as most nutrients become available in this pH range. pH can be determined by mixing a soil sample with water and then measuring the resulting aqueous solution.
Introduction
Soil pH is a measure of the acidity or alkalinity in soils. In the pH scale, pH 7.0 is neutral. Below 7.0 is acidic and above 7.0 is basic or alkaline. Soil pH affects nutrients available for plant growth. In highly acidic soil, aluminum and manganese can become more available and more toxic to plant while calcium, phosphorus, and magnesium are less available to the plant. In highly alkaline soil, phosphorus and most micronutrients become less available.
When designing or planting new garden or landscape, it is helpful to check the levels as different plants thrive in different ranges. pH determination can indicate whether soil is suitable for the plants to be grown or it needs to be adjusted to produce optimum plant growth.
Soil pH can be measured easily and inexpensively at home or on-site using LAQUAtwin pH meters. There are three (3) LAQUAtwin pH meter models available, namely pH 11, 22, and 33. These pocket-sized compact meters allow two to five calibration points using either NIST or USA pH buffers. Among the above-mentioned models, the pH 33 meter has built-in temperature sensor that measures and displays temperature reading and automatic temperature compensation feature (ATC) that performs automatic calibration to the exact pH of the buffer at the measured temperature. Refer to the specifications of each meter model for more information.
Method
Calibrate the LAQUAtwin pH meter according to manufacturer’s instructions using at least two pH buffers that bracket the expected sample pH.
Sample Preparation and Measurement
The method described below is based on US EPA Method 9045D. This is also applicable for measuring pH of waste samples, which may be solids, sludges, or non-aqueous liquids. If water is present, it must constitute less than 20% of the total volume of the sample.
Add 20ml of pure water to 20g sample in a beaker or container. Stir for 5 minutes then cover.
Let the soil suspension stand for about 1 hour. Alternatively, filter or centrifuge off the water phase.
Measure the pH of water phase. Record the pH value and the temperature.
To obtain accurate results, standard buffer solutions and samples should be measured at the same temperature. If the electrode is coated with oily material from a sample, clean it with detergent and warm water.
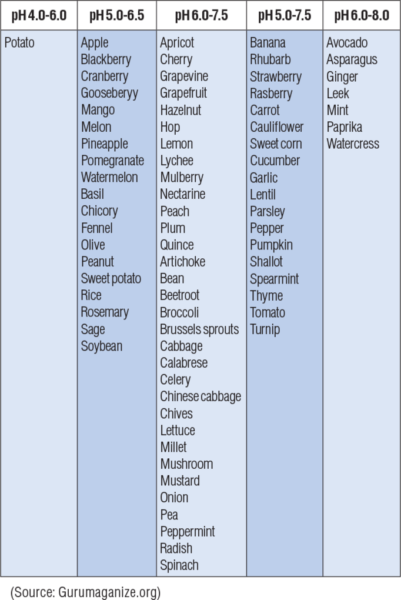
Results and Benefits
The desirable range for optimum plant growth varies among crops. Generally, soil pH 6.0-7.5 is acceptable for most plants as most nutrients become available in this pH range. Soil pH is important because it affects the availability of nutrients to plants. Nitrogen, phosphorus, and potassium are the primary nutrients needed in fairly large quantities. Calcium, magnesium, and sulfur are secondary nutrients required by the plant in lesser quantities. Zinc and manganese are micronutrients required by the plant in very small amounts. Most secondary and micronutrient deficiencies are easily corrected by keeping the soil at the optimum pH value.
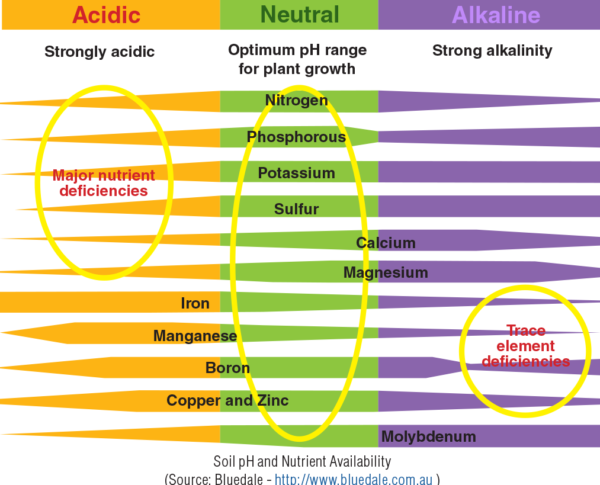
Soil pH also affects activity of microorganisms. The population of bacteria that decompose organic matter declines and their activity is hindered in highly acidic soil, therefore, results in accumulation of organic matter and the bound nutrients, particularly nitrogen.
Increasing Levels
Applying a material that contains some form of lime (calcium carbonate) like the ground agricultural limestone and wood ashes can increase the levels. The finer the limestone, the more rapid it becomes effective. Different soils will require a different amount of lime to adjust the soil pH. Wood ashes contain high amounts of potassium and calcium, and small amounts of phosphate, boron and other nutrients. Although not as effective as limestone, it can drastically increase the soil pH with repeated use.
Decreasing Levels
Aside from ammonium-based fertilizers and organic matter, aluminum sulfate and sulfur are common materials used for decreasing levels. Aluminum sulfate is preferred as it changes the soil pH as soon as it dissolves because of the aluminum. However, too much of this is toxic to plant. Sulfur takes some time to produce effect as it needs to be converted to sulfuric acid by soil bacteria.
References and Suggested Readings
- US Environmental Protection Agency Method 9045D Soil and Waste pH, Revision 4, November 2004
- Changing the pH of your Soil. Clemson University Cooperative Extension. www.clemenson.edu
REV 1.0, 22 SEPTEMBER 2015