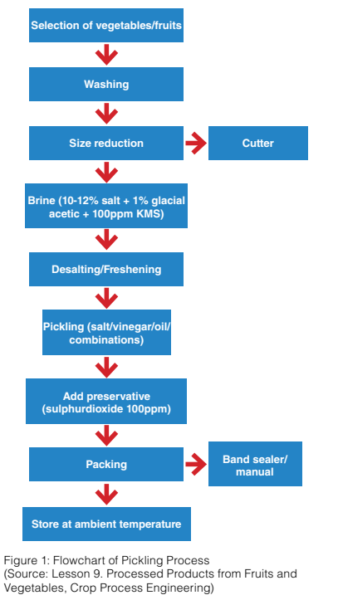
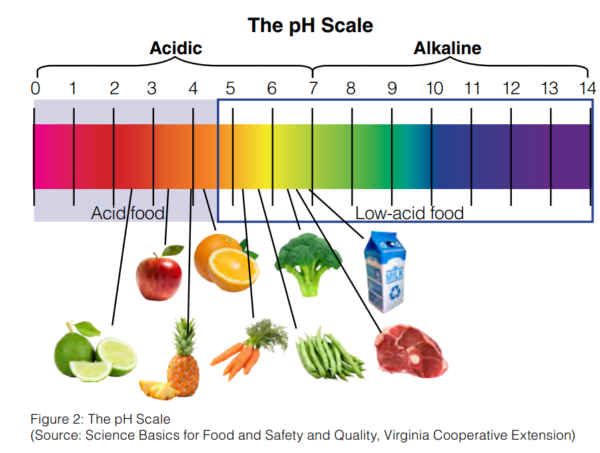
Pickling is a process of preserving fruits and vegetables in brine, oil, water or vinegar. The Australia New Zealand Food Standard Code 2.3.1 requires the preserved fruits and vegetables to have a pH not greater than 4.6 to prevent botulism.
 Introduction
Introduction
Vegetables in oil have caused botulism outbreaks in United States. Botulism is caused by the anaerobic, spore-forming bacterium Clostridium botulinum. This resulted to the development of Title 21 of the Code of Federal Regulation (21 CFR 114), a regulation for acidified foods. Australian authorities adopted similar precautions to 21 CFR 114 and included a requirement in the Australia New Zealand Food Standard Code 2.3.1. This code states that “fruits and vegetables in brine, oil, vinegar or water, other than commercially canned fruit and vegetables, must have a pH not greater than 4.6”.
The LAQUAtwin pH meter have three (3) models, namely pH 11, 22 and 33, which can be used to measure pH of pickled fruits and vegetables. These pocket sized meters allow two to five calibration points using either NIST or USA pH buffers. The pH 33 meter has built-in temperature sensor that measures and displays temperature and automatic temperature compensation feature (ATC) that performs automatic calibration to the exact pH of the buffer at the measured temperature. Refer to the specifications of the meters for more information.

 Method
Method
Calibrate the LAQUAtwin pH meter using pH 4.01 and 7.00 buffers according to manufacturer’s instructions.
Sample Preparation And Measurement
1. Drain the liquid of pickled fruits and vegetables.
2. Blend the fruits and vegetables in a blender to a paste consistency. For some samples, it may be necessary to add a small amount of distilled water (less than 20mL DI in 100g sample) to facilitate blending. This will not alter the pH of most products as distilled water contains no hydrogen ions.
3. Place a portion of the paste into the sensor.
4. Record the pH and temperature once stabilized.
5. After each sample, rinse the sensor with water and blot dry with soft tissue.
6. Determine two pH values on the blended sample. These readings should agree with one another to indicate that the sample is homogeneous.
 Results and Benefits
Results and Benefits
Food acidity is important in preventing botulism, a foodborne illness that comes from eating contaminated food with toxins produced by C. botulinum. This fact is used in preparing pickled fruits and vegetables. Aside from following tested recipes in acidification and proper packaging of products, performing accurate pH measurement using a reliable instrument is also necessary to check if pH 4.6 or below is attained for food safety and regulatory compliance.
References
1. NSW Food Authority. Shelf stable acid preserved foods. NSW/FA/FI035/0811
2. Title 21 of the Code of Federal Regulation (21 CFR 114)
REV 0, 13 AUGUST 2015